Publications Update # 78

Non-Medical Medical Article of the Week
It’s really a Medical article, but it’s bigger than the topics of medicine or gynecology oncology that we discuss. It speaks to those moments of uncertainty—when you’re unsure if you’re making the right call, when you’re in the middle of a meaningful conversation with a patient but are conscious of the clock ticking, or when you’re negotiating with administrators who want to run the cancer clinic like a high-efficiency factory, where volume is prioritized.
The essence of the piece is the human factor in healthcare. Sometimes, what may seem like inefficiency is intentional—it’s part of the design, not a flaw, because providing care is not just about speed or numbers; it’s about the person in front of you.
Cervical Cancer
Video Discussion of Interlace Trial

Study Summary
Hypothesis
Adding induction chemotherapy before standard cisplatin-based chemoradiotherapy improves progression-free survival (PFS) and overall survival (OS) compared to chemoradiotherapy alone in patients with locally advanced cervical cancer.
Inclusion Criteria
- Adults (≥18 years) with FIGO 2008 stage IB1 (with nodal involvement), IB2, IIA, IIB, IIIB, or IVA cervical cancer.
- Histology: squamous, adenocarcinoma, or adenosquamous.
- Fit for radical treatment and no para-aortic lymph node involvement.
Exclusion Criteria
- FIGO 2008 stage IIIA disease.
- Positive lymph nodes above the aortic bifurcation.
Primary End Point
- Progression-free survival (PFS)
- Overall survival (OS)
Experimental Arm(s)
- Induction chemotherapy regimen:
- Carboplatin AUC 2 and paclitaxel 80 mg/m² weekly for 6 weeks.
- Followed by standard chemoradiotherapy starting in week 7.
- Standard chemoradiotherapy regimen:
- Cisplatin 40 mg/m² weekly for 5 weeks.
- External beam radiotherapy (EBRT) 45.0–50.4 Gy in 20–28 fractions.
- Brachytherapy to achieve a minimum total 2 Gy equivalent dose of 78–86 Gy.
Control Arm (or Standard Therapy)
- Standard chemoradiotherapy regimen:
- Cisplatin 40 mg/m² weekly for 5 weeks.
- EBRT 45.0–50.4 Gy in 20–28 fractions.
- Brachytherapy as per the experimental arm.
Results
| Outcome | Induction Chemo + Chemoradiotherapy | Chemoradiotherapy Alone | p-value | Hazard Ratio (HR) |
|---|---|---|---|---|
| 5-year Progression-free Survival (PFS) | 72% | 64% | 0.013 | 0.65 (95% CI 0.46–0.91) |
| 5-year Overall Survival (OS) | 80% | 72% | 0.015 | 0.60 (95% CI 0.40–0.91) |
| Grade 3 or greater adverse events | 147 (59%) | 120 (48%) | - | - |
| Haematological toxicities | 74 (30%) | 32 (13%) | - | - |
Top Toxicities
| Toxicity Type | Induction Chemo + Chemoradiotherapy (n=250) | Chemoradiotherapy Alone (n=250) |
|---|---|---|
| Grade 3-4 Hematological Toxicity | 74 (30%) | 32 (13%) |
| Neutropenia | 48 (19%) | 13 (5%) |
| Anaemia | 13 (5%) | 9 (4%) |
| Thrombocytopenia | 13 (5%) | 5 (2%) |
| Non-hematological Toxicity | 109 (44%) | 107 (43%) |
| Diarrhea | 20 (8%) | 31 (12%) |
| Fatigue, muscle weakness, or joint pain | 28 (11%) | 14 (6%) |
| Infection | 14 (6%) | 13 (5%) |
| Abdominal or pelvic pain | 13 (5%) | 18 (7%) |
Conclusions
Induction chemotherapy followed by chemoradiotherapy significantly improves both progression-free survival and overall survival in patients with locally advanced cervical cancer compared to chemoradiotherapy alone. However, it leads to higher haematological toxicities.
Limitations
- The trial excluded patients with FIGO 2008 stage IIIA or para-aortic lymph node involvement, which limits the generalizability to the highest-risk groups.
- Recruitment took 10 years, and variations in radiotherapy techniques may have affected outcomes. Although its important to recognize that improvements in radiation techniques, such as IMRT improves toxicity compared to standard chemoRT and not the survival.
Ovarian Cancer
Final Survival Data - PRIMA Study

As you might recall, the PRIMA study evaluated the role of Niraparib in patients with newly diagnosed ovarian cancer. The table below shows that the original study highlighted the progression-free survival benefit of 5 months in the overall population and roughly 11 months in the HRD population. The most significant benefit was seen in patients with BRCA mutation.
| Metric | Niraparib Group | Placebo Group | p-value | Hazard Ratio |
|---|---|---|---|---|
| Progression-Free Survival (months, HRD population) | 21.9 | 10.4 | <0.001 | 0.43 |
| Progression-Free Survival (months, overall population) | 13.8 | 8.2 | <0.001 | 0.62 |
| Overall Survival (24-month interim analysis, %) | 84 | 77 | 0.70 | |
| Grade ≥3 Adverse Events (%) | 70.5 | 18.9 |
The current publication, reports the overall survival after a median followup of 6 years.
None of the subgroups showed a survival benefit in the final overall survival data. Few possible explanations:
- Crossover—Almost 36% of the BRCAmut patients in the placebo arm received a PARP inhibitor, which could have blunted the OS effect. In this subgroup, the PFS benefit is substantial.
- It is possible that Niraparib is inferior compared to Olaparib - maybe not its effectiveness per se but its lower tolerability is an issue.
Overall, using PARP-i in patients with HR-d/BRCAwt and those with BRCAmut is still a reasonable strategy. However, the PARP landscape has shrunk over the last few years. Here is approach that I use
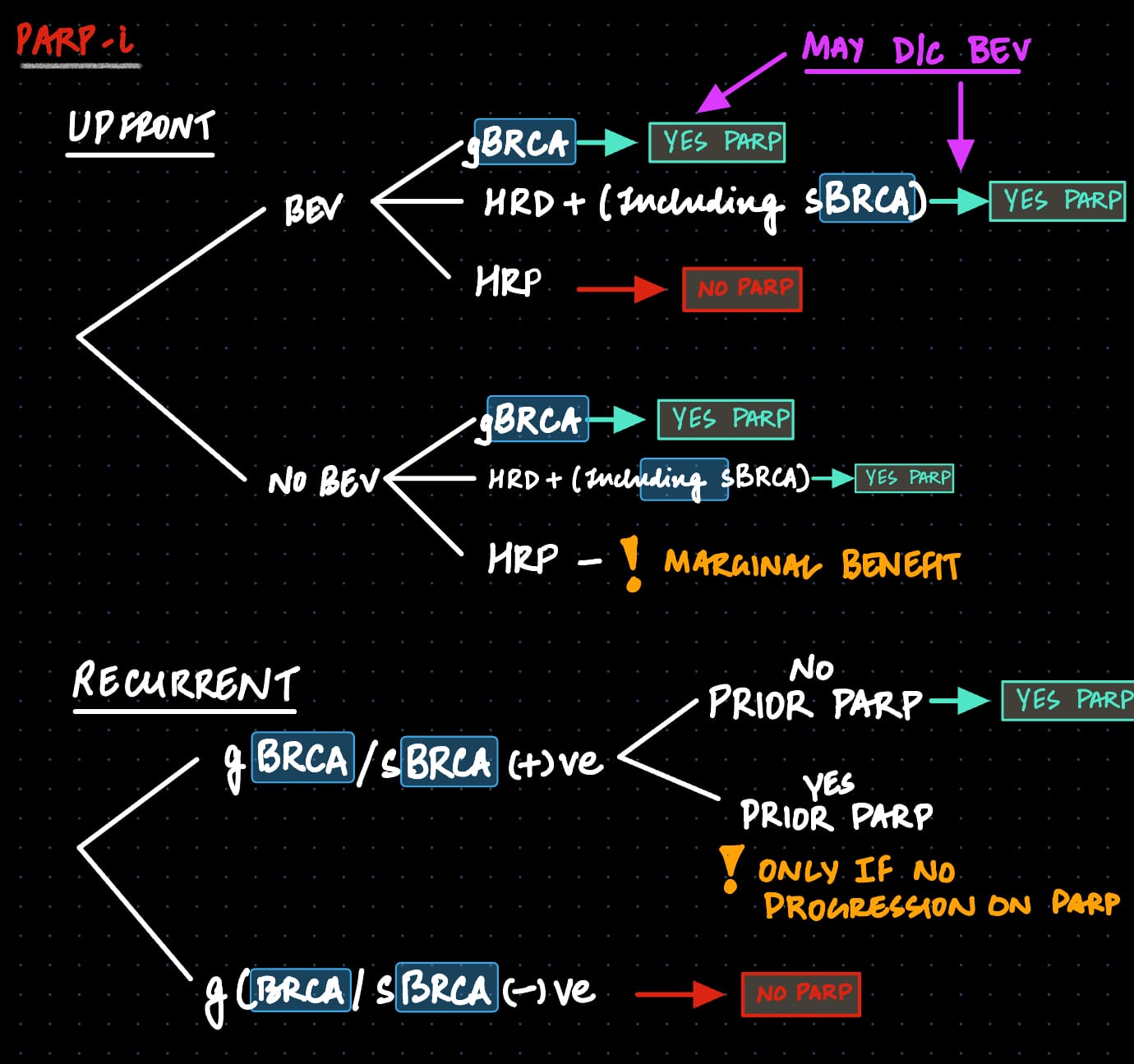
Follow @gyoedu Follow @uppals

